
We have learned a lot about high blood sugar, insulin resistance, inflammation, and its impact on atherosclerosis. All of these are very closely intertwined.
But one of the critical physiological procedures, that remains silently behind these processes, which also needs to be discussed, is called Advanced Glycation End (AGEs) Products.
AGEs are molecules that are formed when a protein binds to a molecule of glucose to form a covalent bond. We can also say that proteins get glycated chemically. Let’s first understand how they’re formed and how they affect us.
Glucose has three major forms. The two most prominent ones are the cyclic forms: alpha isomer and beta isomer. They form a ring-like structure. Therefore, they are also called the cyclic form of glucose. Then there is a very small concentration of the linear or straight chain form, also called the acyclic form, as it doesn’t form a ring.
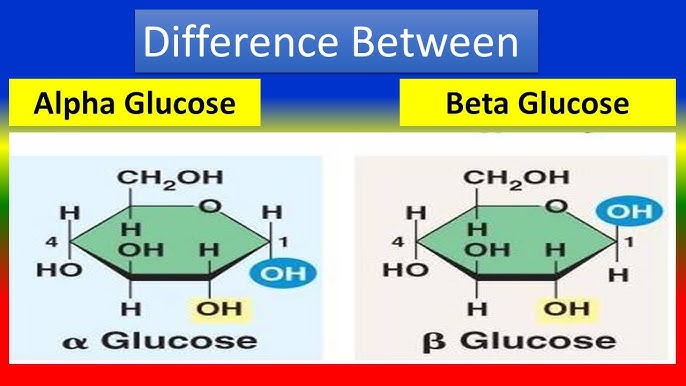
However, in biological systems, the alpha and beta isomers are constantly interchangeable with one another. But for the alpha isomer to get to the beta isomer and the beta isomer to get to the alpha isomer, first it must be recyclized into the linear form. Then it can be recycled into the other cyclic isomer.
At any given time, linear acyclic isomers are very few, but some of these acyclic, linear forms of glucose—the aldehyde functional group—labeled position 1 are very reactive, particularly to the amino acid lysine. When they come into contact, the lone pair of nitrogen in the amino acid lysine can nucleophilically attack the carbon of that aldehyde, and this forms a covalent bond.
The next step is a Schiff base formation, an intermediate rearrangement of the double bonds between nitrogen and carbon. Double bond formation results in the loss of one (H2O) water molecule.
There is no requirement for any enzyme in this process, which happens spontaneously. Therefore, these are non-enzymatic reactions.
The last step is the Amadori rearrangement, which is again a rearrangement of the bonds. There is a single nitrogen-carbon bond and a carbon-oxygen double bond, and this is the most stable of all these forms.
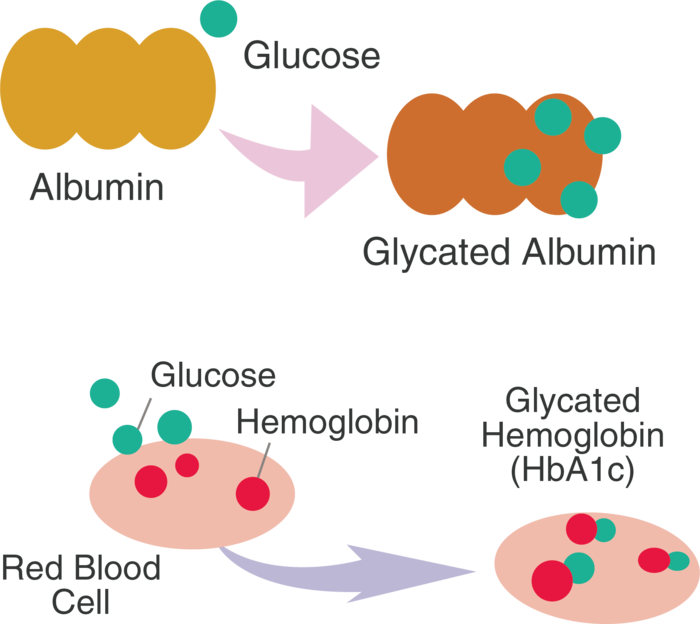
The protein here is different, as it is now chemically bonded to glucose (glycated).
Why is this Relevant?
- Number one: This new molecule, a form of the original protein, is now bound to the glucose molecule, and it cannot reverse itself to its original structure. It’s an irreversible reaction.
- Number two: This new molecule, where we have a protein-glucose adduct, is termed AGEs.
There is always glucose in the blood at any given time. Every single person has blood glucose; that’s what our cells run on. With normal, healthy blood glucose, in a healthy individual, the AGE formation process happens only to a small extent. There are mechanisms in place within our body to get rid of these AGEs and keep them at bay. It is the extent of their formation that dictates health versus disease.
In a healthy individual, the fasting blood sugar value should be somewhere between 70 and 100 mg/dL. That’s a low and healthy value. Therefore, the number of AGEs that will form will be very small, and they’re not going to produce harmful effects in the body as our body has mechanisms in place to counteract them.

However, when their production becomes excessive and exceeds the body’s ability to get rid of them, we start seeing their accumulation and their physiological effects.
Diabetes and AGEs
Hyperglycemia, or chronic hyperglycemia, is a condition with a lot of blood glucose; that’s what we see in individuals who consume high-sugar diets and people with type 2 diabetes.
So, with excess sugar in the blood, there is excess glycation of proteins. You get an accumulation of these AGEs.
What are Their Biological Effects?
RAGE stands for “Receptor of the Advanced Glycation End product.” These receptors are present in certain immune cells, nerve cells, bone tissue, and lung cells.
We’re going to look at two major pathways.
This is a macrophage, and in the plasma membrane, it has a protein called RAGE. AGEs can bind to this protein, and when they do, it activates certain pathways inside the cell.
The first pathway we’re going to look at is the NOX pathway. NOX is a membrane-bound protein called NADPH phagosome oxidase.
The NADPH phagosome oxidase enzyme is a committed step in reactive oxidative species (ROS) synthesis.
But for the NADPH phagosome oxidase enzyme to get activated, AGE must bind to its RAGE receptor. And when that happens, an intracellular signalling cascade occurs. Immediately, the activation of NOX NADPH phosome oxidase takes place.
This enzyme catalyzes the irreversible conversion of molecular oxygen (O2) into the superoxide radical, which is a free radical. It is a reactive oxidative species.
Recall the body’s 3 enzymatic natural antioxidants: SOD (Superoxide Dismutase), Catalase, and Glutathione.
The superoxide radical can be converted to hydrogen peroxide by SOD (superoxide dismutase). The resulting hydrogen peroxide is not a free radical, but it is a Reactive Oxidative Species (ROS).
Hydrogen peroxide can react in one of two ways. First, it can react with the antioxidant enzyme catalase. Catalase is known as a protective antioxidant enzyme. So, catalase will get rid of hydrogen peroxide by converting it into water and molecular oxygen, both of which are harmless.
Alternately, hydrogen peroxide can react with iron in the ferrous state, the Fe+2 state. Fe+2 can reduce hydrogen peroxide into a molecule of water and a hydroxyl radical.
Hydroxyl radicals are extremely dangerous. They’re the most reactive of any of the reactive oxidative species. Hydroxyl radicals can damage membrane lipids, damage DNA, damage proteins, and cause cellular damage.
In addition, they can also oxidize and damage LDL particles. And these oxidized LDLs can cause a tremendous amount of damage, especially when their production is excessive.
So, activation of this NOX enzyme pathway will lead to the production of superoxide, hydrogen peroxide, and hydroxyl radicals.
It’s also worth noting that LDLs can also be glycated by glucose and themselves be AGEs, which can also activate the RAGE receptor and move in a vicious cycle.
These glycated and oxidized LDLs that contribute to atherosclerosis are going to be taken up by macrophages, which causes them to release inflammatory cytokines and differentiate into foam cells, which contributes to plaque formation.
_1730717295.jpg)
The second pathway of the AGEs is NF-kappa B.
We have an AGE that binds to its receptor, RAGE, and RAGE can indirectly activate NF-kappa-B.
RAGE activates a protein called IKK. IKK is a kinase protein that phosphorylates certain proteins. Inside, we have a complex protein called I kappa B alpha. I kappa B alpha inhibits the function of RelA and p50.
The two proteins (RelA and p50) combined are NF-kappa B. As long as kappa B alpha is bound to these two proteins, it keeps them inhabited. So, when RAGE activates IKK, IKK phosphorylates I kappa B alpha, which loses grip on RelA and P50 and floats away. The two phosphates are then degraded via the proteasomal pathway, leaving free RelA and p50.
Collectively, these two proteins are NF-kappa B, which itself is a transcription factor. It comes into the nucleus through the nuclear pore complex and binds to specific elements in the DNA.
NF-kappa B requires other proteins, like a co-activator and RNA polymerase. It also recruits RNA polymerase to a target gene, resulting in its transcription into mRNA. Then mRNA leaves the nucleus and enters the cytoplasm, where it’s picked up by ribosomes and translated into a protein.
Conclusion
It all started with AGEs, which activated RAGE. Do you think it’s going to be pro-inflammatory or anti-inflammatory? Well, it’s going to be pro-inflammatory. So, what we see here is that the AGEs bind to RAGE, which activates the NF-kappa B pathway, which changes gene expression. This results in the production of inflammatory cytokines and a whole host of such proteins. They also trigger the production of all sorts of other inflammatory molecules, including oxidized LDL. These inflammatory cytokines and oxidized LDL are going to contribute to inflammation and the progression of atherosclerosis.

.png)


