
In both skeletal muscle and the brain, diabetes induces mitochondrial dysfunction, including decreased mitochondrial respiration, reduced oxidative phosphorylation, and increased oxidative stress. Diabetes also interferes with stem cell function.
Diabetes mellitus is one of the most common metabolic diseases spread all over the world and results in hyperglycemia caused by the breakdown of insulin secretion, insulin action, or both. Diabetes has been reported to disrupt the functions and dynamics of mitochondria, which play a fundamental role in regulating metabolic pathways and are crucial to maintaining appropriate energy balance. Similar to mitochondria, the functions and abilities of stem cells are attenuated under diabetic conditions in several tissues.
In recent years, several studies have suggested that the regulation of mitochondrial functions and dynamics is critical for the precise differentiation of stem cells. Importantly, physical exercise is very useful for preventing diabetic alteration by improving the functions of both mitochondria and stem cells.
Diabetes Mellitus
Diabetes mellitus (DM) is one of the most common metabolic diseases worldwide, and the number of patients with diabetes mellitus has continued to increase in recent years. Patients with diabetes mellitus exhibit hyperglycemia caused by an impairment in insulin secretion type 1, insulin action type 2, or both. Type 1 diabetes mellitus, which accounts for less than 10% of diabetes cases, is characterized by an immune-mediated destruction of β cells in the pancreatic islets of Langerhans, leading to insulin deficiency. It is well known that T1DM is developed in childhood and can lead to severe long-term complications, including retinopathy, neuropathy, and nephropathy. On the other hand, type 2 diabetes mellitus (T2DM), which accounts for less than 90% of diabetes cases, involves insulin resistance in peripheral tissues and increased levels of blood glucose due to overnutrition accompanied by deficient insulin secretion. DM is often associated with the development of secondary complications in various organs, such as the eyes, kidneys, heart, brain, and skeletal muscle.
Mitochondria and stem cell dysfunctions are among the multiple factors that can cause disturbances to the skeletal muscle and nervous system functions in DM. Mitochondria play critical roles in regulating metabolic pathways and maintaining appropriate energy balance in tissues. DM is associated with reduced mitochondrial function, including decreased mitochondrial numbers, impaired lipid oxidation, and excessive production of reactive oxygen species (ROS). The proliferation and differentiation of skeletal muscle stem cells, termed satellite cells, are attenuated in diabetic skeletal muscle. Moreover, the proliferative ability of neural stem cells (NSCs) is declining in the hippocampus of T1DM animal models. The neurogenesis of NSCs is impaired in DM because of decreased expression of the transcription factor NeuroD1. These mitochondrial and stem cell dysfunctions may disrupt cell homeostasis, resulting in the disturbance of skeletal muscle and brain function in DM.

Mitochondria Dysfunction in Diabetes
Skeletal muscle is highly plastic tissue that can adapt to changes in energy status via changes in mitochondrial content. Previous studies examining the relationship between mitochondria and insulin resistance have reported that the skeletal muscles of patients with T2DM exhibit reduced mitochondrial content. Mitochondrial oxidative capacity is significantly lower in the skeletal muscle of insulin-resistant individuals than in healthy subjects, and this alteration results in increased fat accumulation in the skeletal muscle. Disturbed mitochondrial function has also been observed in cultured myocytes derived from the skeletal muscle of patients with T2DM.
Mitochondrial content is controlled by mitochondrial biogenesis, which is induced by various physiological, environmental, and pharmacological stimuli through the promotion of several regulators.
Muscle Stem Function in Diabetes
Resident satellite cells in skeletal muscle contribute to the postnatal maintenance, growth, repair, and regeneration of skeletal muscle. In healthy adult muscle, satellite cells are mitotically quiescent under normal physiological conditions but are activated in response to stimulation, such as muscle injury, to become myoblasts and proliferate extensively. Most proliferated myoblasts then undergo myogenic differentiation to fuse with existing fibers or generate new muscle fibers. In contrast, others return to a quiescent state to self-renew and maintain the stem cell pool. Satellite cell-depleted mice exhibit poor muscle regeneration after muscle injury, suggesting satellite cells are essential for muscle regeneration.
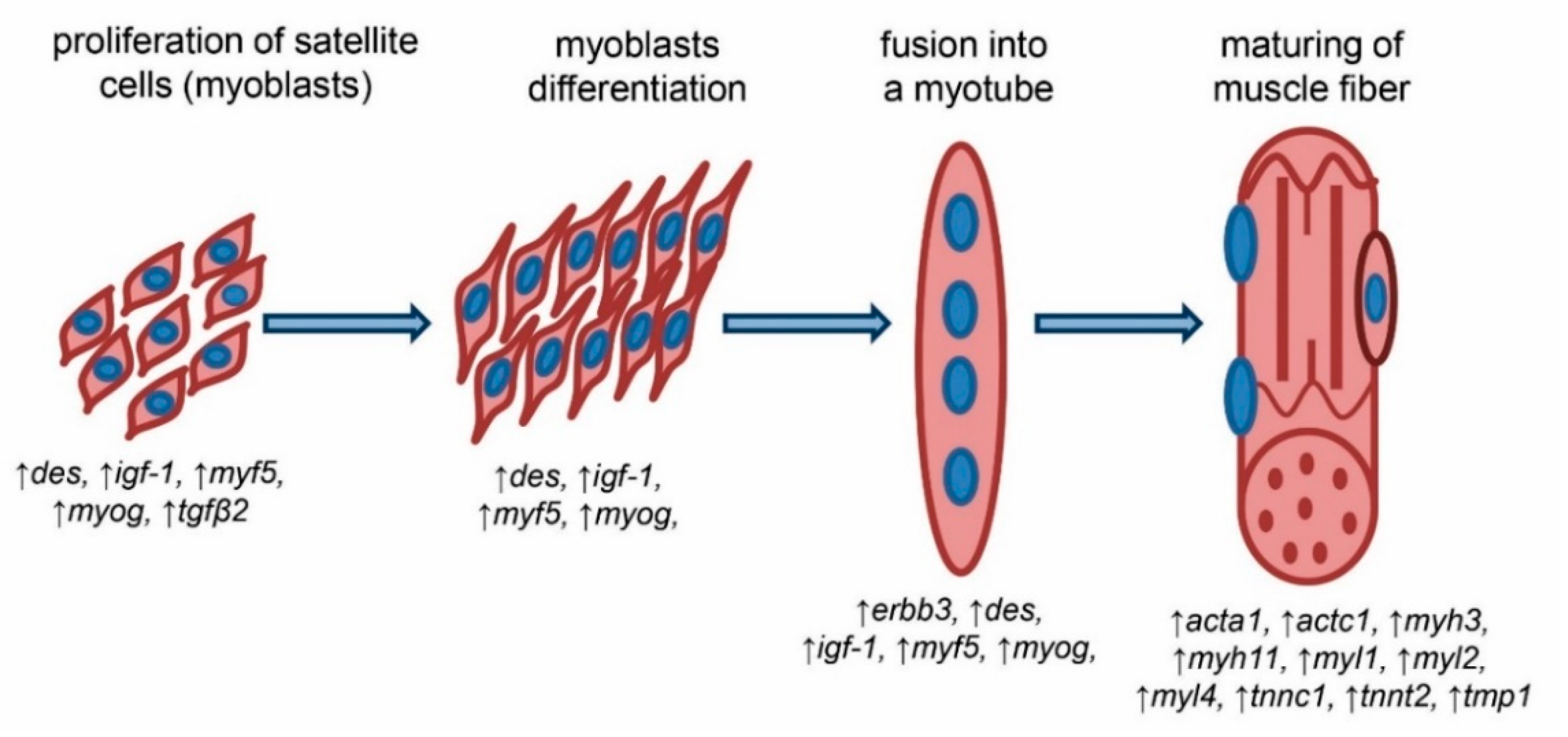
Satellite cells demonstrate at least two states in skeletal muscle turnover: a quiescent state and an activated state. Both quiescent and activated satellite cells express the characteristic marker Pax7, whereas only activated satellite cells also express Myf5 and MyoD, which are key transcription factors for myogenic lineage progression and differentiation. Previous studies have shown that DM impairs satellite cell function. Satellite cells derived from STZ-induced diabetic mice are unable to form my tubes, resulting in poor regeneration after cardiotoxin-induced muscle injury.
Impairment of Neural Stem Function
Neurogenesis in the adult mammalian brain is a multistep process, including the proliferation of neural progenitor cells, fate determination, migration, neuronal maturation, and functional integration of newly born cells into the existing neuronal circuitry. NSCs are primarily located in two distinct regions of the brain: the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone of the hippocampal dentate gyrus (DG).
In the SVZ, adult NSCs give rise to neuroblasts, which migrate into the olfactory bulb (OB) through the rostral migratory stream and then differentiate into mature local interneurons. In the DG, proliferating neuroblasts become immature neurons and project their axons into the CA3 region of the hippocampus. These immature neurons eventually differentiate into mature neurons and are integrated into the existing hippocampal circuitry as functional granule cells. Recent studies have shown that newly formed neurons are incorporated into the functional networks of both the OB and the DG, suggesting that adult neurogenesis notably affects brain functions associated with learning, memory processing, and odor discrimination.
Preventive Measures of Physical Exercise on Diabetes
Physical exercise can change mitochondrial content, shape, and function in skeletal muscle. Exercise boosts the creation of new mitochondria in skeletal muscle. Endurance exercise promotes this process through repeated training sessions. The protein PGC-1α, which increases with exercise, plays a key role in this mitochondrial growth. Both short-term and long-term exercise raise PGC-1α levels in muscle, but it is not fully understood if this increase is essential for the creation of new mitochondria.
Exercise improves the quality of mitochondria in skeletal muscle. Resistance training boosts the levels of proteins like MFN1, MFN2, and OPA1, which help mitochondria fuse. Endurance swimming has a similar effect. Exercise also raises the levels of Fis1 and activates DRP1, which increases mitochondrial splitting. Exercise and muscle contractions increase ROS production and oxidative stress. While too much ROS can harm muscle proteins and organelles, a moderate amount is essential for muscle signaling and balance. Studies show that mice given antioxidants had poor mitochondrial function and couldn't tolerate exercise well, highlighting the importance of balanced ROS for healthy cells.

Conclusion
In conclusion, diabetes disrupts mitochondria and stem cell functions in skeletal muscles and the nervous system, impacting tissue health and regeneration. Physical exercise emerges as a crucial intervention, enhancing mitochondrial biogenesis and quality control while mitigating oxidative stress, thereby offering potential benefits for managing diabetes-related complications and improving overall health outcomes.
Frequently Asked Questions (FAQs)
1. How does diabetes affect mitochondrial function in the body?
Diabetes impairs mitochondrial function by reducing mitochondrial respiration, decreasing oxidative phosphorylation, and increasing oxidative stress, which leads to disrupted energy production and metabolic imbalances in various tissues.
2. What specific changes occur in the mitochondria of individuals with diabetes?
In individuals with diabetes, mitochondria exhibit decreased numbers, impaired lipid oxidation, and excessive production of reactive oxygen species (ROS), contributing to cellular damage and metabolic dysfunction.
3. How does diabetes influence stem cell function in skeletal muscles?
Diabetes attenuates the function and proliferation of satellite cells, which are muscle stem cells essential for muscle repair and regeneration, leading to poor muscle maintenance and recovery.
4. Can physical exercise help counteract the effects of diabetes on mitochondria and stem cells?
Yes, physical exercise can enhance mitochondrial biogenesis, improve mitochondrial quality control, and activate stem cells, thereby mitigating the adverse effects of diabetes on these cellular components.
5. How does diabetes-induced stem cell dysfunction affect tissue repair and regeneration?
Diabetes-induced stem cell dysfunction limits the ability of tissues to repair and regenerate after injury, leading to prolonged recovery times, chronic tissue damage, and increased susceptibility to complications.

.png)


