Insulin is increasingly being recommended as an oral therapy for the achievement of blood glucose in people with type 2 diabetes. Insulin has an unlimited potential to lower blood glucose and is a well-established treatment for type 2 diabetes. IDF recommends that it be used as an optional third line when a combination of metformin, where indicated, and one other glucose-lowering medication has failed to adequately control blood glucose. The ADA and EASD guidelines recommend a patient-centered approach to achieve adequate glucose control while minimizing side effects.
Insulin has an unlimited potential to lower blood glucose and is a well-established treatment for type 2 diabetes. Insulin is an established glucose-lowering therapy for both type 1 and type 2 diabetes. However, insulin is a growth factor. It is administered in an unphysiological manner, and it is present in the circulation at levels that are far higher than in people without diabetes. For these reasons, concerns regarding insulin safety have been long-standing, and this has led to them being extensively investigated and, to my mind, repudiated.
Managing type 2 diabetes is complex and necessitates careful consideration of patient factors such as engagement in self-care, comorbidities, and costs. Since type 2 diabetes is a progressive disease, many patients will require injectable agents, usually insulin.
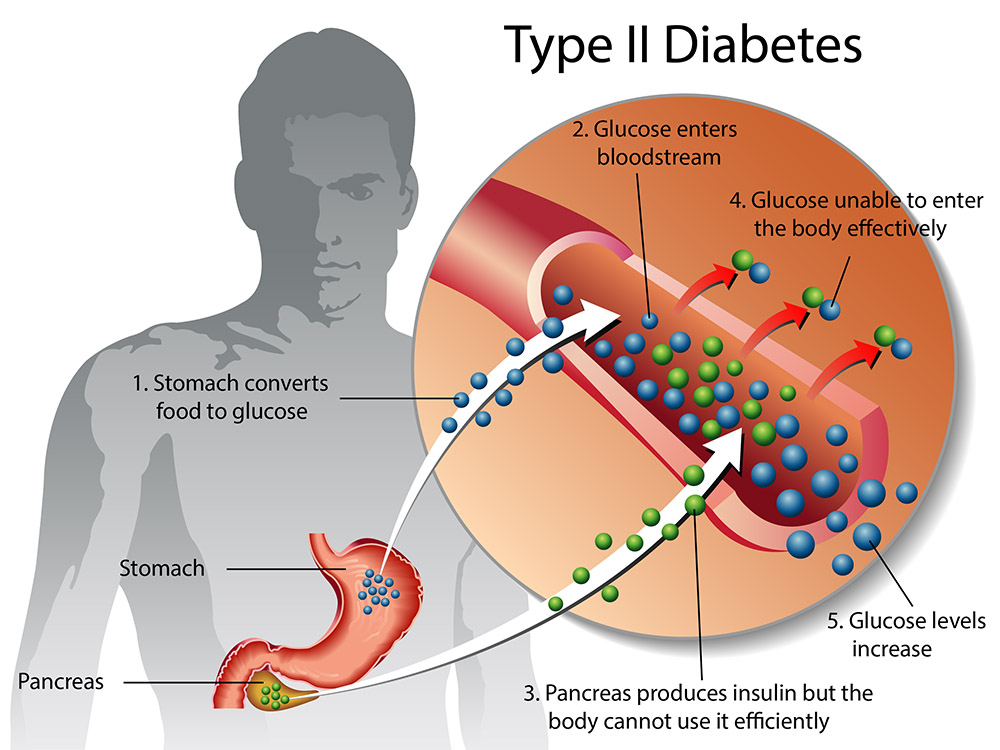
Management of type 2 diabetes is complex, and quality care necessitates careful consideration of patient factors such as preferences, engagement in self-care, comorbidities, and costs. The goals of diabetes care include preventing diabetes-related complications and maintaining or enhancing quality of life. Since type 2 diabetes is a progressive disease, achieving these goals will often require intensification of injectable therapy. Classically, this has been accomplished with insulin.
Anxiety and Depression and the Role Played by the Gut Microbiome
It is now a common belief that the gut microbiota communicates with the central nervous system through neural, endocrine, and immune routes and thereby controls brain function. Studies have demonstrated a substrain role for the gut microbiota in the regulation of anxiety, mood, cognition, and pain.
The role of the microbiome and microbiota-derived molecules in regulating such interactions is unclear; however, the mechanisms underpinning such effects are only beginning to be resolved. Microbiota-gut peptide interactions are poised to be of great significance in the regulation of gut-brain signaling. Given the emerging role of the gut-brain axis in a variety of brain disorders, such as anxiety and depression, it is important to understand the contribution of bidirectional interactions between peptide hormones released from the gut and intestinal bacteria in the context of this axis. Gut peptides in the systemic circulation can bind cognate receptors on immune cells and vagus nerve terminals, thereby enabling indirect gut-brain communication. Gut peptide concentrations are not only modulated by enteric microbiota signals but also vary according to the composition of the intestinal microbiota. In this review, we will discuss the gut microbiota as a regulator of anxiety and depression and explore the role of gut-derived peptides as signaling molecules in microbiome-gut-brain communication.
Gut Microbiota
The diversity and stability of the microbiota are important indices for the overall health of an individual. The gut microbiota may impact host health via the conversion of non-digestible carbohydrates to short-chain fatty acids (SCFAs), the transformation of bile acids' action against pathogenic bacteria, and the modulation of host innate and adaptive immune systems.
Microbiota-Gut Brain Pathways
The brain and the gut are engaged in continuous, bidirectional communication. Such communication may be important in mediating physiological effects ranging from GI function to the brain and behavior, bringing about the perception of visceral events such as nausea, satiety, and pain. Gut-brain communication may begin as sensory information from the GI tract and is consequently transformed into neural, hormonal, and immunological signals. These signals can independently or cooperatively relay information to the central nervous system (CNS). Peptides are short chains of amino-acid monomers linked by amide bonds (approximately ≤ 50 amino-acid residues) that can be released from specialized neurons in the brain (where they are called neuropeptides) or in EECs as a signaling molecule through binding G protein-coupled receptors. Many of the peptides found in EECs and in the CNS regulating the GI tract are also found in the enteric nervous system.
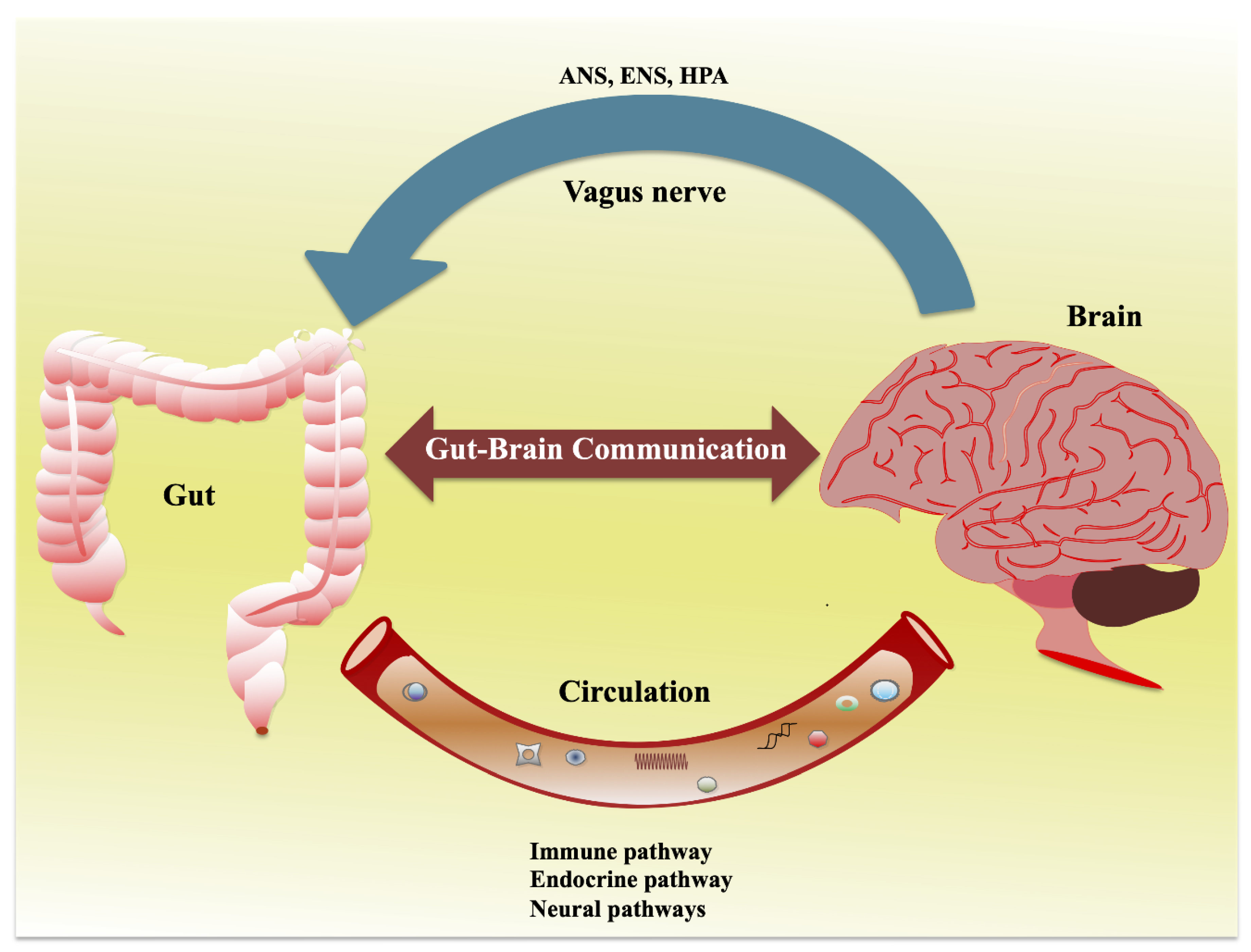
Gut Microbiota in Anxiety and Depression
Depression and anxiety are often accompanied by changes in colonic motility, which, in turn, alters the composition and stability of the gut microbiota as well as the colonic physiology and morphology. Stress-related disorders can also alter the intestinal barrier, triggering a “leaky gut," which could allow a microbiota-driven proinflammatory response through the translocation of certain bacterial products from the gut. The study failed to identify correlations between anxiety or depression symptoms and scores.
Similar observations have been made in animal studies. Found that depressive-like rats had the relative abundances of Bacteroidetes significantly increased, whereas that of Firmicutes was markedly decreased. In mice that underwent chronic stress, reductions in the genus Bactericides were identified. Stressed mice have also been shown to have greater populations of the genus Clostridium, which agrees with the gut microbiota profile in maternally separated rodents. The genus Clostridium is commonly altered as a result of changes modulated by gut metabolites such as phenylalanine, tryptophan, and tyrosine. Such metabolites are part of the metabolism of key neurotransmitters in mammals, including serotonin, with implications for ENS and CNS function and thus gut-brain axis signaling. Certain antibiotics, such as β-lactams and tetracyclines, have potential antidepressant properties in rodents and humans. Epidemiologic studies have suggested that some classes of antibiotics, such as fluoroquinolones, are associated with the development of depression and anxiety.
The gut microbiota and its effects on host metabolic phenotype during psychiatric illness remain unknown, but these observations highlight the potential association of the gut microbiota with host depression and anxiety. Thus, the manipulation of the gut microbiota may be a useful tool to decode the physiological role of the gut-brain axis in psychiatric disorders. The use of germ-free rodents, animals with pathogenic bacterial infections, animals exposed to probiotic agents or antibiotics, prebiotics, and fecal microbiota transfer from another animal or human may lead to important new findings for the development of new therapeutic strategies to treat or prevent anxiety and depression.
Role of Epigenetics in Diseases Development and Prevention
Epigenetics and Development
Epigenetic changes begin before you are born. All your cells have the same genes but look and act differently. As you grow and develop, epigenetics helps determine which function a cell will have, for example, whether it will become a heart cell, nerve cell, or skin cell. It is correlated with many human diseases, including different cancers, autoimmune disorders, neurological disorders (Fragile X syndrome as well as Huntington's, Alzheimer's, and Parkinson's diseases), and schizophrenia.
Epigenetic modifications have a considerable effect on cancer. Hypermethylation of promoter regions in tumor suppressor genes can inactivate many tumor suppressor functions. Methylation levels also play an important role in cell divisions, DNA repair, differentiation, apoptosis, angiogenesis, metastasis, growth factor response, detoxification, and drug resistance. Such features have promoted huge advances in the early detection of cancer using methylation levels. There are also some other types of epigenetic changes in cancer. In recent years, deregulation of miRNAs has been confirmed in breast cancer, which has the potential to be used as diagnostic biomarkers. Also, hyper- and hypo-methylation of several genes in breast cancer have been confirmed.
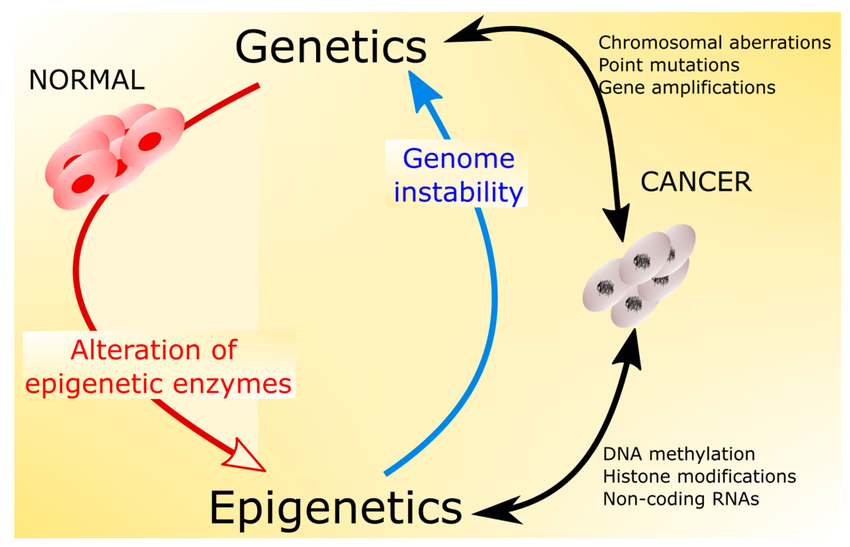
Natural and normal functions of the immune system depend on self-tolerance, and self-tolerance deficiency can result in autoimmunity. Autoimmune disease concordance studies in both monozygotic and dizygotic twins have suggested a role for epigenetic factors.
Epigenetic homeostasis failure, as a response to environmental agents, can result in gene expression changes in specific differentiated cells, leading to deregulated self-tolerance.
The immune system and target organ are two main players in an autoimmune disease process, and the epigenetic modifications of these players could have roles in disease development. Many functions of immune cells, such as hematopoietic lineage, rearrangement of antigen- receptors, allelic exclusion, and inducible immune responses against pathogens, are epigenetically controlled. The alterations in epigenetic mechanisms regulating immunological development could promote autoimmunity disease.
The frequency of autoimmune disease occurrence is notably higher in women, and the reason may be due to female sex hormones. The involvement of the second X chromosome in immune response and genetic predisposition to autoimmunity is suspected. Considering the lack of enough knowledge on the exact cause of these immune diseases, a role for epigenetic regulatory mechanisms is highly possible. There are many examples of correlations between epigenetic modifications and autoimmune diseases.
Several studies have confirmed the role of epigenetics in allergic conditions, and asthma is considered one of the most complicated diseases in this category. Evidence suggests that both asthma and epigenetic mechanisms are heritable, and 36–79% of heritable, familial asthma cases have a non-Mendelian inheritance pattern in more than 100 genes, which covers only a small portion of the disease etiology. Interestingly, asthma and epigenetic modifications are transferred from the affected mother more than the affected father in parental origin features, which can be a result of immune interactions between the fetus and the mother. Utero exposures can affect asthma as well as epigenetic modifications, and both features can be influenced by environmental factors.
The Link Between Gut Dysbiosis and Autoimmune Diseases
Autoimmune diseases occur when the immune system mistakenly produces antibodies that attack the body's own tissues. Genetic and environmental factors play roles in triggering this abnormal immune response, which leads to the production of autoantibodies and inflammatory molecules. Changes in gut bacteria composition, known as dysbiosis, are thought to contribute to autoimmune diseases by affecting how the immune system functions. This can happen through various mechanisms such as molecular mimicry, altered gut barrier function allowing bacteria to interact with immune cells, and triggering abnormal immune responses. These interactions highlight the potential impact of gut health on autoimmune disease development and progression.
Conclusion
In summary, insulin remains crucial for managing type 2 diabetes when other treatments fail. The gut microbiota plays a significant role in influencing anxiety, depression, and autoimmune diseases through complex interactions with the brain and immune system. Understanding these connections could lead to new therapies for improving mental health and combating autoimmune conditions.
Frequently Asked Questions (FAQs)
1. How does the gut microbiota affect mental health?
The gut microbiota communicates with the brain through various pathways, influencing mood, cognition, and stress responses. This connection underscores the importance of gut health in mental well-being.
2. Can epigenetic changes be reversed to treat diseases?
While epigenetic modifications are influenced by environmental factors, ongoing research investigates whether targeted interventions can reverse these changes to mitigate disease risks or improve treatment outcomes.
3. What are some practical ways to support gut health?
Consuming a diverse diet rich in fiber, probiotics, and prebiotics can promote a healthy gut microbiota. Lifestyle factors like stress management and regular exercise also play crucial roles.
4. Are there natural alternatives to insulin therapy for type 2 diabetes?
While insulin is a standard treatment, ongoing research explores natural compounds and lifestyle modifications that may help manage blood glucose levels, complementing conventional therapies.
5. What is the future direction of research in insulin therapy and gut microbiota interactions?
Future studies may focus on personalized approaches to insulin therapy based on individual gut microbiota profiles, aiming to optimize treatment efficacy and minimize side effects.

.png)


